Our mission is to care about your breath, our vision to combine Human Tech with Human Touch... You just feel free to breathe.

Oxygen, nitrogen and carbon dioxide are a few examples: their high kinetic energy produces random movements, giving this substance the advantage of moving from one place to another without hindrance.
Acetone, toluene and hexane are some examples. Another example occurs when water is boiled: in this case it is called water vapour.



Inertisation limits the action of oxygen inside the system to prevent the formation of explosive atmospheres. Inertisation units are used when it is necessary to avoid:
If materials are processed, whose exhalations combined with oxygen in the air result in the formation of an easily flammable mixture, any possible risk of explosion must be prevented. In practice, air is replaced by inert gas.
Nitrogen, argon and helium are some examples and present some peculiar risks:
A substance that conveys a pungent, recognisable odour is generally added to the gases, thus increasing the level of safety for those exposed.

However, in the presence of inert gases and oxygen deficiency (less than 18%), inhaled air ceases to be breathable to the point of causing asphyxiation and death to those exposed to it.

Chlorine, phosgene, sulphur dioxide, hydrochloric acid, hydrogen sulphide, nitrogen dioxide, ozone and ammonia are among the most important irritant gases. Hydrogen sulphide is also a potent cellular toxin, which blocks the cytochrome system and inhibits cellular respiration.
A common exposure involves domestic mixing of ammonia with detergents containing bleach; this releases chloramine (NH2Cl), a highly toxic gas that when it comes into contact with mucous membranes decomposes and generates hydrochloric acid, which is toxic and corrosive, and free radicals, which are carcinogenic.
Acute exposure to toxic gases generally characterises industrial accidents, which occur as a result of the release of large quantities of the substance over a short period of time.
In the environments of this sector, it is possible for gases such as carbon dioxide, nitrogen and argon to be released and the related risk of asphyxia to occur. Asphyxiation appears to be the leading cause of death in this type of confined space accident.
Oxygen consumption can occur while carrying out activities in confined spaces in the absence of adequate indoor air renewal.
To date, the toxic gases recognised as such under RD No. 147/1927 are as follows:
To select the right protection, it is necessary to know the chemical nature of the pollutant by ensuring that the oxygen level is above 17%.
For more information on the nominal protection factor (FPN) and the PPE selection read the last paragraph of our article on Health Risks of Welding: PPE selection.
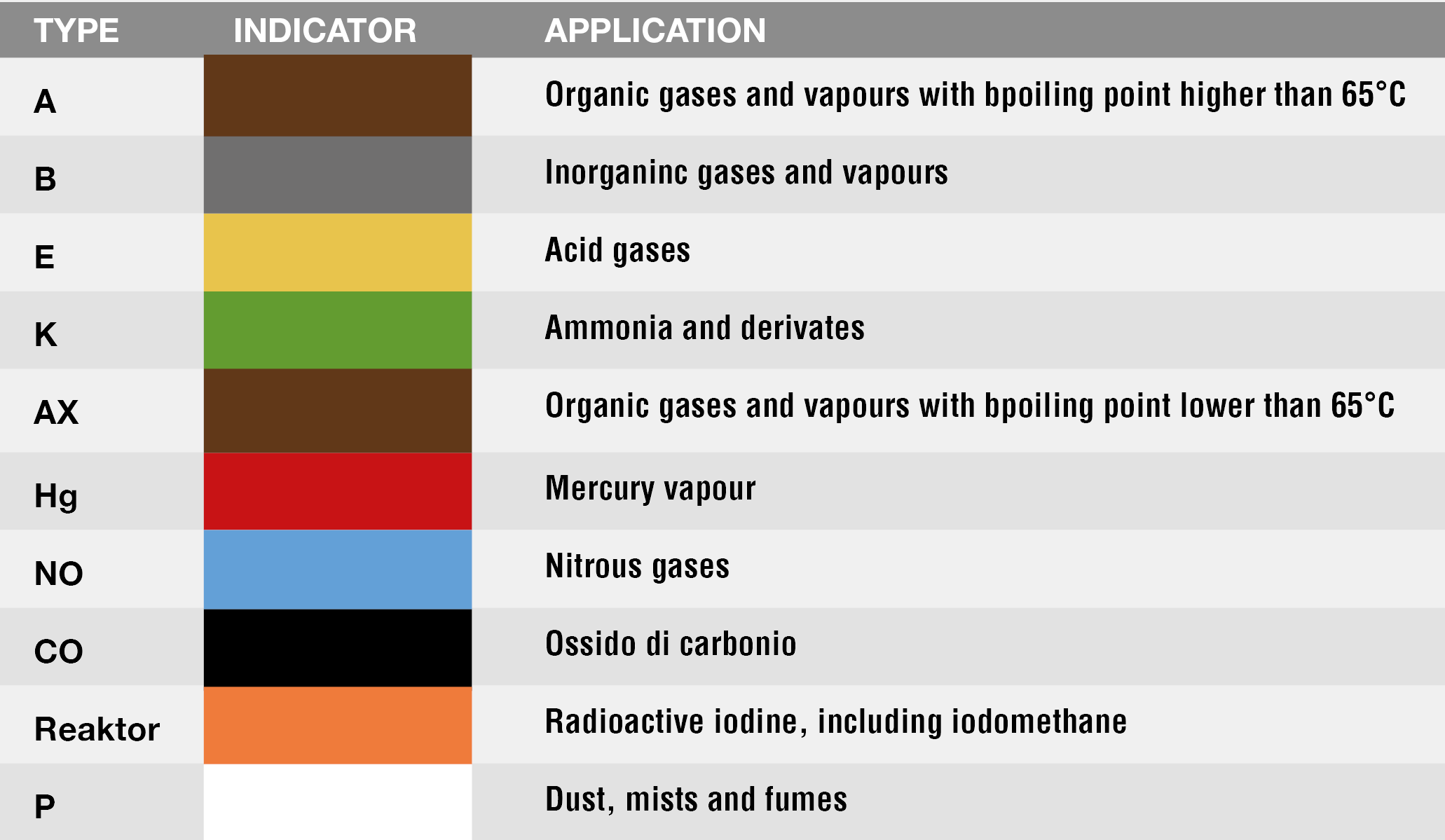
The European Standard EN 14387:2004+A1:2008 defines protection against gases and vapours according to the following table:
The products that BLS offers you to protect your health from toxic gases and vapours are half masks and full face masks with a wide range of models that vary in terms of type of connection, materials and method of use.
Single-filter products are associated with the BLS 400 universal connection filter series. Bi-filter products are associated with the BLS 200 filter series with b-lock connection, the bayonet connection made by BLS.


Our mission is to care about your breath, our vision to combine Human Tech with Human Touch... You just feel free to breathe.
Sai indossare correttamente un DPI? Indossare un DPI nel modo corretto è fondamentale per trarne massima efficacia e migliori performance di prodotto. Lo scopo della protezione respiratoria individuale è proteggere chi la indossa da sostanze tossiche o patogene diffuse per via aerea. Il suo utilizzo, quindi, dovrebbe essere preceduto da una formazione appropriata sulle […]
Cosa sappiamo sui batteri patogeni? I batteri patogeni costituiscono tra le forme viventi più diffuse sulla Terra. I batteri patogeni sono responsabili in tutto il mondo di malattie e conseguenze anche su scala epidemica. Si propagano attraverso l’aria, l’acqua e il suolo. Organismi a cellula procariota, solitamente unicellulari, oscillano tra 0,2 e 10 µm e […]