Pharmaceutical Industry
Essential guide to protecting workers’ health and supporting process continuity
The pharmaceutical industry is one of the most highly regulated and complex sectors from a safety perspective.
The manufacturing of pharmaceutical products involves the use of numerous chemical substances obtained through fermentation, organic synthesis or biological processes. These substances are often handled at very high concentrations and may pose risks to the health and safety of workers.
In an environment where precision, compliance and risk mitigation are uncompromisable standards, choosing the correct respiratory protective device is never just a detail: it is an essential element to ensure quality, efficiency and safety throughout all stages of production.
Recurrent operations
The main processes typical of the pharmaceutical sector expose personnel to different types of contaminants, which must be thoroughly assessed to identify the most suitable respiratory device.
- Granulation and mixing
Processes that may generate fine dusts, aerosols and solvent vapours during the preparation or finishing of active ingredients. - Handling, use or storage of chemicals (acids, bases, solvents)
Activities involving direct exposure to corrosive, irritant, toxic or sensitising substances. - Cleaning and sterilisation of environments, equipment and containers
Operations requiring the use of disinfectants and volatile solvents, which may release harmful vapours. - Weighing of raw materials
One of the most delicate phases, during which the dispersion of active ingredient particles can easily occur.
Main risks
Exposure to airborne particulates
Very fine dusts, solid or liquid aerosols generated during mixing, milling or distribution.
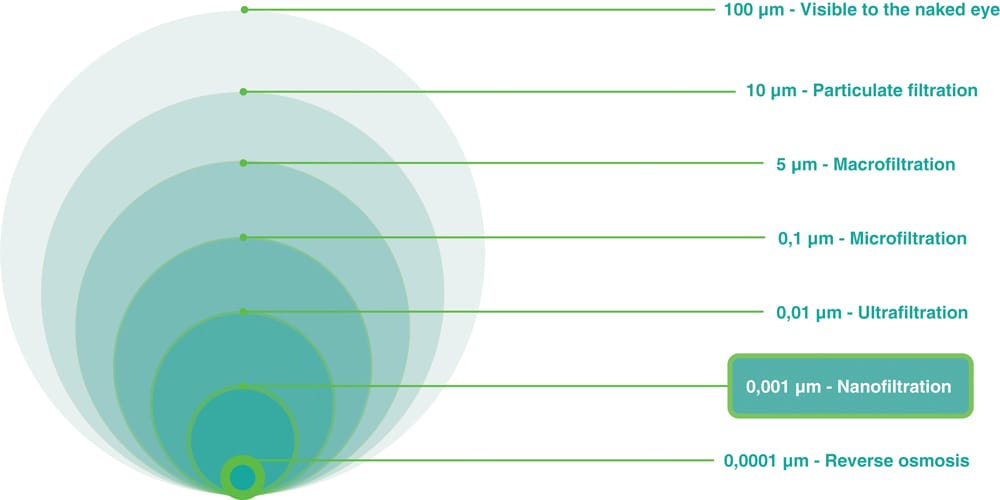
In this context, nanofiltration is of particular importance, as it is essential for capturing particles smaller than 0.1 microns—typical of many pharmaceutical active ingredients.
Studies show that certain nanoparticles can penetrate cells and tissues, move throughout the body and the brain, and cause biochemical damage. Due to their extremely small size, nanoparticles often behave in a way that is intermediate between gases and suspended particulates.

Acute and chronic exposure to chemical substances
Inhalation, accidental ingestion or contact with toxic, neurotoxic, sensitising or carcinogenic compounds typical of synthesis and transformation processes.
Vapours and aerosols during cleaning or sterilisation operations
Solvents, detergents and disinfectants can release irritant vapours and residual particles, especially in closed or poorly ventilated environments.
To explore the full range of risks associated with different processes, consult our complete Safety Guide.
To learn more about all the risks associated with different processes, please consult our comprehensive Safety Guide.

The right products for each process
Selecting the appropriate respiratory protective device requires two fundamental evaluations to be carried out in sequence:
1. Nature and toxicity of the contaminant
The nature of the contaminant refers to whether it is present in solid form (dusts, fumes, aerosols) or as a gas/vapour.
For solid contaminants (dusts, fumes, aerosols), the options include:
filtering facepieces (FFP),
half masks or full face masks with particulate filters,
Powered Air Purifying Respirators (PAPR).
Considering the earlier discussion on nanoparticles (i.e., particles smaller than 0.1 microns), products integrating nanofiltration technology are recommended.
The BLS Zero range offers superior filtration performance, designed to capture ultrafine particles and ensure advanced protection in the most sensitive applications.
The same nanofiltration performance is also available in BLS particulate filters and combined filters, for both half masks and full face masks, developed to address the specific needs of pharmaceutical manufacturing areas.
For exposure to gases and vapours, filtering facepieces are not suitable; instead, one must opt for:
2. Concentration of the contaminant
The second step consists in selecting the device based on its protection factor, considering the Nominal Protection Factor (NPF) defined by EN 529 and, where applicable, the Assigned Protection Factors (APF) used in individual countries.
In many situations, filtering facepieces or reusable devices may be replaced by PAPR systems, whose advantages include:
no breathing resistance;
reduced perspiration and increased comfort;
no compatibility issues with prescription glasses;
suitable for users with facial hair;
guaranteed protection without the need for fit testing.

BLS offers a comprehensive range of PAPR systems designed to meet the needs of drug and active ingredient production, including a version suitable for ATEX environments, where safety and electrostatic discharge control are essential requirements.
BLS Team is available to support companies and operators in identifying the most appropriate solution for each specific risk scenario. For further guidance on choosing the right devices, please contact us.

Good habits for safe and effective use
The effectiveness of a respiratory protective device does not depend solely on the device itself, but also on its correct use. To maintain high performance and ensure proper protection, it is essential to:
Wear the device before entering the contaminated area and remove it only at the end.
Follow the donning instructions carefully. Video tutorials are available on the BLS YouTube channel.
Check proper fitting with a seal check every time the mask is worn, ensuring perfect facial adherence.
Follow cleaning and maintenance checklists according to the instructions in the user manual to preserve device functionality.
Provide dedicated storage areas for respiratory devices.
Verify compatibility with other PPE (e.g., hearing protection). Improper combinations may compromise the face seal.
Inspect the device for cracks, holes or damage. Deformations or visible wear may compromise the seal.
[Read more in the dedicated article]
Comfort and safety during long shifts
In pharmaceutical environments, work shifts can be long and require constant concentration and full adherence to procedures. In this context, comfort becomes a key factor—not only for the operator’s wellbeing but also for ensuring the quality and continuity of the production process.
- Selecting protection proportional to the risk: Maximum protection is not always the most appropriate choice. Overprotection may result in heavier or more demanding devices, directly impacting comfort and operator performance. The selection must therefore balance safety, ergonomics and ease of use.
- Breathing resistance matters: devices that require less effort for inhalation and exhalation allow workers to perform tasks for longer without fatigue, especially when the operational load is high.
To respond to this need, BLS has developed BLS Zer0 32 Active, the first FFP3 filtering facepiece with Active Shield technology.
The Active Shield is an innovative extraction valve featuring an adaptive fan that reduces humidity and heat, removes CO₂ and significantly enhances operator comfort while extending the filtering facepiece’s lifespan.
Technologies such as Active Shield significantly contribute to reducing the perception of breathing effort, making the use of PPE more bearable even for those who are prone to greater fatigue
- For reusable devices, the material of the facepiece plays a key role. The availability of TPE or silicone models allows you to choose the most suitable solution depending on the specific risk situation; soft silicone in particular reduces the risk of irritation or discomfort during long hours of continuous use.
Silicone is a very soft material that is pleasant to the touch. It has important characteristics of non-deformability and resistance to high temperatures, and has greater resistance to the aggression of organic compounds, such as solvents. TPE has a slightly less soft surface, but is easily mouldable, making it more economical and easier to recycle. It also has excellent resistance to acids and bases.
[Read more in the dedicated article]
- Finally, it is essential to consider the variety of physiognomies. Specific facial conformations or particular needs require the availability of alternatives that still guarantee effective protection without compromising the fit or increasing the ineffectiveness of the device. A wide range and the availability of multiple sizes for some products allows you to find the most suitable PPE for each operator, ensuring comfort and safety throughout the entire shift.
[Read more in the dedicated article]
Safety in pharmaceutical processes also depends on the careful selection and proper use of respiratory protective devices. By accurately assessing the nature of the contaminant, choosing the appropriate device for each production phase and maintaining good operational practices, it is possible to ensure protection, continuity and comfort even in the most sensitive and regulated environments.
BLS is by your side with products and guides for effective and comfortable protection, always.
Stay informed
Stay safe.